[:de]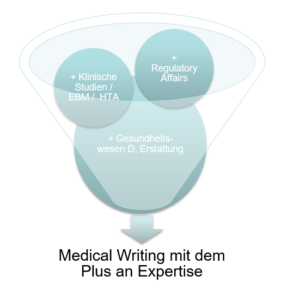
Das Medical Writing mit dem Plus an Expertise
Was ist das Plus an Expertise?
Das Plus an Expertise hilft, dass Ihr wertvolles Projekt möglichst schnell und treffsicher den maßgeschneiderten Rahmen findet.
Wenn zum Beispiel die Ergebnisse aus dem Risikomanagement in die klinische Bewertung mit aufgenommen werden sollen. Dann ist es ein großer Vorteil, wenn Ihr Medical Writer auch das Risikomanagement beherrscht. Er kann dann zusätzlich beratend zur Seite stehen und dafür sorgen, dass alles passt.
Oder, wenn im Medical Advisory Board über Erstattungsprobleme diskutiert wird. Dann ist es schön, wenn Ihr Medical Writer auch versteht, wie das Gesundheitssystem funktioniert. Dann wird die Diskussion verstanden und gleich im ersten Anlauf richtig zu Papier gebracht.
Vielleicht geht es aber auch um die Veröffentlichung von Studiendaten? Dann ist es recht elegant, wenn Ihr Medical Writer tatsächlich selbst schon Teil von klinischen Studienprojekten war. Er weiß dann einfach besser Bescheid, worauf es ankommt.
Woher kommt das Plus an Expertise?
- Die solide Grundlage: Dr. rer. nat. – Diplom Biologin
- Weiterbildung: Regulatory Affairs Manager für die Medizintechnik (Zertifikat Uni Lübeck)
- Weiterbildung im Marketing
- Weiterbildungen in der Wissenschaftskommunikation
- Zertifiziertes Know-how in Statistik (Duke University)
- Erwerb von fundierten Kenntnissen der evidenzbasierten Medizin
- Longstanding industry experience in the subject areas Medical Affairs/ Regulatory Affairs
- Erfahrung durch eigenes Projektmanagement klinische Studien (GCP)
- Erfahrung durch zahlreiche eigene, unabhängige und erfolgreiche Medical-Writing Projekte
- Klinische Bewertungen Meddev 2.7.1
- Erstattungsanträge
- Veröffentlichungen
- Kongress-Berichte
- Study Write-Ups
- …
- Und sonst noch: Deutsch (Muttersprache), Englisch (Europäischer Referenzrahmen C2), Französisch (B2), Italienisch (B2), Finnisch (A1)
Sie möchten mehr wissen?
Rufen Sie uns gerne an oder schreiben Sie uns eine Email!
Klinische Daten für die Marktzulassung und Erstattung, Klinische Bewertung MEDDEV 2.7.1
Original-Publikationen, Abstracts, klinische Prüfpläne, Buchkapitel, Protokollführung Medical Advisory Boards
Newsletter, Kongressberichte, redaktionelle Beiträge, Internet-News.
[:en]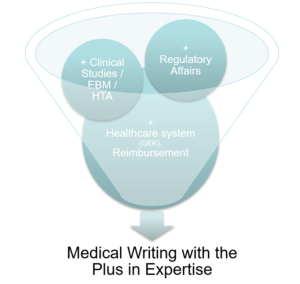
Medical Writing with the Plus in Expertise
What is the plus in expertise?
The plus in expertise helps your valuable project find its made to measure frame in a fast and effective way.
When, for example, the results of the risk management are to be integrated into the clinical evaluation. In that case it proves a great advantage to have a medical writer who is also well-versed in risk management. He can then take on an additional advisory function to make sure everything’s just right.
Or when reimbursement is being discussed in the Medical Advisory Board. In that case it is nice to have a medical writer who understands the way the healthcare system works. He can then follow the debate and correctly commit it to paper at the first go.
Or maybe it is about publishing study data instead? In that case it is quite elegant to have a medical writer who has actually been a part of clinical study projects himself. He is then simply better at knowing what really matters.
How do we get the plus in experience?
- The solid foundation: Dr. rer. nat. (PhD) – academically qualified biologist
- Advanced training: Regulatory Affairs Manager Medical Devices ( Certified by Universität zu Lübeck)
- Advanced training in marketing
- Advanced training in scientific communication
- Certified training in statistics (Duke University)
- Profound knowledge of evidence-based medicine
- Longstanding industry experience in the subject areas Medical & Regulatory Affairs
- Experience through own project management of clinical studies (GCP)
- Experience through numerous own, independent and succesful Medical Writing projects
- Clinical Evaluations Meddev 2.7.1
- Reimbursement applications
- Publications
- Congress reports
- Study write-ups
- …
- And also: German (native), English ( Common european framework of reference level C2), French (B2), Italian (B2), Finnish (A1)
Want to know more?
Just give us a call or write us an email!
Clinical data for the accreditation and reimbursement, clinical evaluation MEDDEV 2.7.1
Original publications, Abstracts, Clinical investigation plans, Book chapters, Minute-taking at Medical Advisory Boards
Newsletters, congress reports, editorials, internet news
[:]
